Fifteen year-old-boy has been complained of intermittent fetid yellow discharge from the right ear canal past one year, he was brought in to the emergency department became of progressive headache and dizziness for the last four days. On initial physical exam, he was alert, cooperative and oriented, but acutely ill looking. At the time of the admission, temperature was found 36.5ºC, blood pressure at 100/60 mm/Hg, and heart rate at 64/min. On neurological examination, he had dysdiadokokinesia, dysmetria on finger to nose test, and signs of meningeal irritation. A fetid discharge mixed with debris was present in the right external ear canal. After the removal of the discharge, right eardrum was inspected. It was macerated and bulged outside. Physical and neurological examination findings were otherwise normal.
In laboratory parameters, hemoglobin was 11g/dL, white blood cell count 11.000/mm3 (80% PNL, 14% lymphocytes, 6% monocytes), platelet count 263.000/mm3, erythrocyte sedimentation rate (ESR) 86 mm/h, CRP 46 mg/L, and ASO 1/400 IU/mL. On differential count, no toxic granulations were observed. Postero-anterior plain radiography of the chest x-ray was unremarkable.
Posterior fossa CT scans without contrast revealed a 2x5 mm lesion of air density located in the right cerebellar hemisphere and it was consistent with the pneumocephalus (Figure 1).
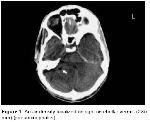
Click Here to Zoom |
Figure 1: An air density localized on right cerebellar vermis (2X5 mm) (pneumocephalus) |
A contrast CT a day later revealed an enhanced 8X4 mm ill-defined lesion compressing the fourth ventricle was demonstrated as well as pneumocephalus that was consistent with early cerebritis stage. An inflammation in mastoid was present which was filling out mastoid antrum destructing the wall and thereby extending into the posterior fossa. The air density (pneumocephalus) was observed adjacent to the mastoiditis lesion (Figure 2).
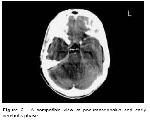
Click Here to Zoom |
Figure 2: A compatible view of pneumocephalus and early cerebritis phase |
Empirically, cefotaxime 3X2 g IV, ampicillin 4x3 g IV and metronidazole 4X500 mg/day IV therapy was implemented, and the patient underwent right radical mastoidectomy. Purulent material provided from the sigmoid sinus during the operation, which was analyzed bacteriologically, and proved the origin of Proteus mirabilis and any bacteria were obtained at anaerobic culture. Because the isolated microorganism was found to be sensitive to cefotaxime, ampicillin, therefore, cefotaxime was continued and ampicillin and metronidazole were discontinued. Drowsiness, meningeal irritation signs, and bradycardia developed on the postoperative 5th day.
The follow-up contrast CT revealed, a biloculated mass lesion (daughter abscess) of 4x4 cm, which was thought to be consistent with cerebellar abscess, was found in the right cerebellar region extending to the tentorium and compressing the medulla posteriorly. Pneumocephalus, which was observed on the previous images, did not persist (Figure 3).
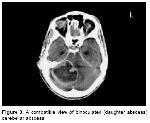
Click Here to Zoom |
Figure 3: A compatible view of binoculated (daughter abscess) cerebellar abscess |
An emergency aspiration was undertaken through a right occipital burr-hole under general anesthesia, and about 40 mL of thick yellow pus was evacuated. The patient developed neither postoperative complications nor fever. Contrast CT taken on 10th postoperative day demonstrated residual abscess formations in 4x3 and 2x1.5 cm diameters, which were extending from right pontocerebellar cistern to the occipital lobe. Fourth ventricle compression was still present. The patient was treated medically and observed clinically. On 33rd day of hospitalization, follow-up contrast CT displayed focal heterogeneous enhanced areas at the level of right cerebellar hemisphere, which were considered as residual inflammatory regions. The ventricular system and Sylvian fissures appeared normal (Figure 4).
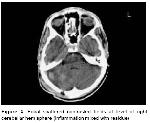
Click Here to Zoom |
Figure 4: Focal scattered contrasted fields at level of right cerebellar hemisphere (inflammation mixed with residue) |
At the end of the 6-week therapy, the patient was clinically stable, and radiologically cleared from abscess formation. The white blood cell count was 7600/mm3, sedimentation rate 25 mm/h, and CRP 6 mg/L. Considering the clinical improvement, the patient was discharged with a prescription of ampicillin 4x2 g P.O. and with cefuroxime axetil 250 mg 2x1 P.O and instructed to come back at the end of 2 weeks of medical therapy.
At first month follow-up, no recurrence or residue was observed on CT scans, but hypodense postoperative changes at the site of previous abscess formation were observed (Figure 5). The physical and neurological findings of the patient were in better state at the first and 3rd day from the operation.
Click Here to Zoom |
Figure 5: Postoperative hypodens changes at the localization of old abscess |



